On February 20, 2023, Astellas ("Astellas") announced that the FDA notified a three-month extension of the PDUFA target date for fezolinetant to May 22, 2023, to allow additional time to complete the review. The company expressed confidence in the clinical performance of fezolinetant and its potential benefit for women experiencing moderate to severe vasodilatory symptoms (VMS) due to menopause.
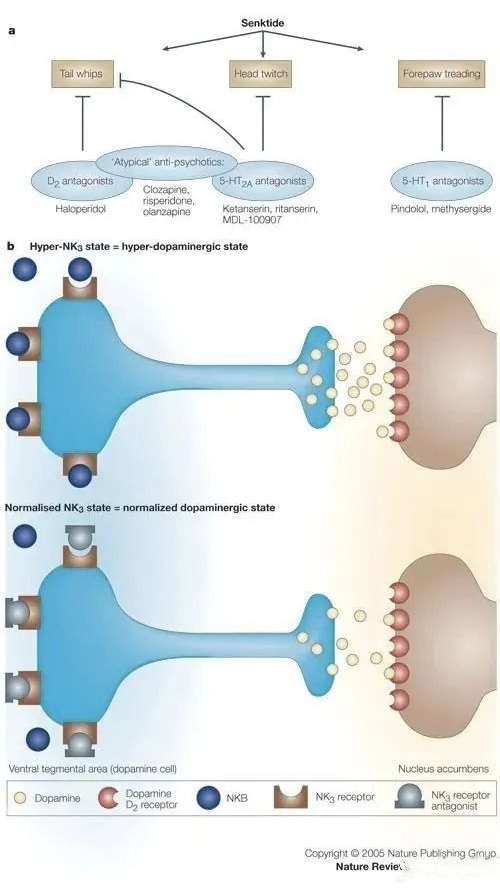
Fezolinetant is an oral, non-hormonal, selective neurokinin 3 (NK3) receptor antagonist for the treatment of menopause-related moderate-to-severe VMS, which modulates neuronal activity in the thermoregulatory centre of the brain (hypothalamus) by blocking the binding of neurokinin B (NKB) to kisspeptin/neurokinin/kynurenine (KNDy) neurons in order to reduce the incidence of menopause-related moderate-to-severe VMS. In 2017, Astellas acquired Ogeda for €500 million (US$534 million) to acquire this drug. Based on sales forecasts, fezolinetant sales are expected to be as high as US$1.5 billion in 2027.
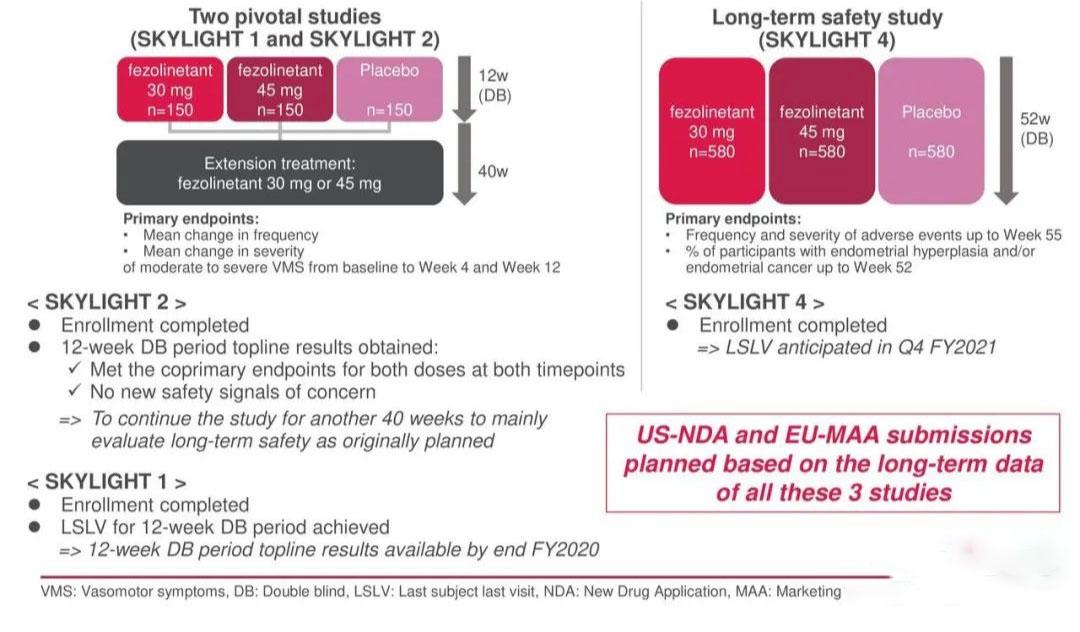
In August 2022, the FDA accepted a New Drug Application (NDA) for fezolinetant for the treatment of moderate to severe menopause-related VMS. if approved, fezolinetant will be the first-in-class non-hormonal treatment option to reduce the frequency and severity of menopause-related VMS. the FDA accepted the NDA for fezolinetant for the treatment of menopause-related VMS. The NDA for fezolinetant was based on the results of two pivotal Phase 3 clinical trials, SKYLIGHT 1 and SKYLIGHT 2, and a Phase 3 long-term safety study, SKYLIGHT 4.
.jpg)
SKYLIGHT 1 and SKYLIGHT 2 were double-blind and placebo-controlled studies conducted in 307 countries and territories in the US, Canada and Europe to assess efficacy in female patients with moderate to severe vasodilatory symptoms. Results showed that both pivotal trials met four primary co-endpoints, with female subjects receiving 30mg and 45mg daily doses of fezolinetant achieving statistically significant reductions in the frequency and severity of moderate to severe VMS from baseline to week 4 and week 12 compared to the placebo group. In terms of safety, a total of no more than 2% of subjects experienced a serious treatment emergency adverse event (TEAE), with the most common side effect manifesting as headache.
SKYLIGHT 4 is a randomised, placebo-controlled, double-blind phase 3 clinical trial in over 1,800 women to validate the long-term (52-week) safety of fezolinetant in menopause-related VMS. The primary objective of this study was to assess the effect of fezolinetant on endometrial health as well as long-term safety and tolerability. The primary endpoints for assessing endometrial health were achieved, with the most common TEAEs being headache and COVID-19, consistent with placebo.
In September 2022, the Company announced positive results from the Phase III MOONLIGHT 3 clinical trial in mainland Chinese women evaluating the long-term safety and tolerability of 30 mg of fezolinetant administered once daily. The frequency and severity of AE, the primary endpoint of the study, were generally consistent with the results of previous Phase III studies of fezolinetant. It is worth noting that in an earlier late-stage MOONLIGHT 1 trial in Asian patients, fezolinetant efficacy was not shown to be superior to placebo.
In October 2022, the company announced that the EMA had accepted a marketing authorisation application (MAA) for fezolinetant. In the marketing authorisation application, Astellas proposed daily dose of 45mg, which will be subject to review by the EMA.
VMS is the most common menopause-related symptom, affecting more than 50% of women aged 40 to 64. The onset of symptoms may precede the last menstrual period, with a median duration of up to 7.4 years. However, one third of women continue to experience moderate to severe hot flushes for more than a decade after the onset of menopause. Hot flushes are a subjective sensation of intense internal heat associated with a range of objective symptoms that include profuse sweating, vasodilation of the skin and a subsequent drop in core body temperature due to a rapid and excessive heat dissipation response.
The main treatment for VMS is still hormone replacement therapy (HRT). Estrogens can be used alone or in combination with progestins, and more than 10 hormone-based monotherapy and combination therapies have been used for this condition. Although effective and generally safe in the short term, long-term use of hormones is associated with an increased risk of venous thromboembolism and should be used with caution in patients at high risk of cardiovascular disease and certain malignancies. In addition, a proportion of patients receiving hormone deprivation therapy as a cancer treatment are not suitable for HRT.
.jpg)
In terms of alternative therapies, there is a limited range of drugs available. Selective 5-hydroxytryptamine reuptake inhibitors (SSRI) and selective 5-hydroxytryptamine and norepinephrine dual reuptake inhibitors are effective in relieving VMS, but can only be used in women with contraindications to HRT. In July 2013, the FDA approved Noven's SSRI Brisdelle (paroxetine) for the treatment of vasodilatory symptoms in women with moderate to severe menopause. Originally an antidepressant, paroxetine also became the first non-hormonal drug to be approved by the FDA for the treatment of VMS in menopausal women.
.jpg)
There has been significant interest in NK3 receptor antagonists as potential therapeutic targets for VMS, but no NK3 receptor antagonists have yet been marketed. Interestingly, these compounds have unique chemical structures at the molecular level with no obvious similarities, yet many have shown efficacy in improving the frequency, severity and quality of life of hot flush symptoms. Moreover, these effects are rapid in onset and last for several weeks compared to conventional HRT.
| Drug name | Research and development institutions | Highest development stage | Target Points |
| fezolinetant | Ogeda(Astellas Pharma) | Application for listing | NK3 |
| elinzanetant | KaNDy | Phase III Clinical | NK1,NK3 |
| Therapeutics(Bayer) NeRRe Therapeutics,GSK | |||
| CS-003 | Daiichi Sankyo | Phase II Clinical | NKl,NK2,NK 3 |
| SJX-653 | Sojournix | Phase I Clinical | NK3 |
| pavinetant | Millendo Therapeutics(Tempest Therapeutics), AstraZeneca | Phase I Clinical | NK3 |
| Thanetan | Smith Kline Beecham(GSK) | Phase I Clinical | NK3 |
| OXANETAN | Acer Therapeutics,Sanofi | Phase I Clinical | NK3 |
| MLE-301 | Millendo Therapeutics(Team pest Therapeutics) | Phase I Clinical | NK3 |
| SAR 102779 | Sanofi | Phase I Clinical | NK2NK3 |
| SSR 146977 | Sanofi | Phase I Clinical | NK3 |
| GSK 172981 | GSK | Preclinical | NK3 |
| GSK 256471 | GSK | Preclinical | NK3 |
| R1551 | Roche | Preclinical | NK1,NK3 |
| RO4583298 | Roche | Preclinical | NK1,NK3 |
| SB218795 | Smith Kline Beccham(GSK) | Preclinical | NK3 |
| SB222200 | Smith Kline Beccham(GSK) | Preclinical | NK3 |
| SB235375 | Smith Kline Beccham(GSK) | Preclinical | NK3 |
| SB-400238 | Smith Kline Beccham(GSK) | Preclinical | NK2,NK3 |
| SB-414240 | Smith Kline Beccham(GSK) | Preclinical | NK2,NK3 |
| SCH 206272 | Schering-Plough(Merck&Co) | Preclinical | NKL,NK2,NK3 |
| SSR241586 | Sanofi | Preclinical | NK2,NK3 |
Please contact us to remove any infringement.