Jul.03.2023
Despite its natural advantages in terms of safety and selectivity, the peptide's Achilles' heel (originally the heel of Achilles, which became his only weakness as it was the only part of his body not immersed in the waters of the River Styx). Achilles was later killed by a poisoned arrow in his ankle during the Trojan War. (now derived as a fatal weakness, a vital point) lies in its poor drug-like properties, susceptible to enzymatic degradation, renal clearance and rapid receptor-mediated clearance, resulting in a short plasma elimination half-life. In addition, parenteral administration causes discomfort and inconvenience to patients, which may negatively affect compliance and ultimately limit therapeutic efficacy.
In a recent article, Derivatization with fatty acids in peptide and protein drug discovery, Nature Reviews Drug Discovery has developed a number of techniques to compensate for these shortcomings. peptide and protein drug discovery [1].
Physiological lipidation itself is a ubiquitous and diverse post- and co-translational process involving various types of fatty acids linked to proteins in different ways, thus providing unique properties to the protein.
Fatty acid-derived subcutaneously injected peptide drugs may remain at the injection site for longer because the fatty acid side chains reduce the rate of diffusion at the injection site, thanks to the binding of fatty acids to the plasma membrane and to albumin (albumin, a large multifunctional transporter protein) in the injected portion. Due to its abundance, long half-life and its function in binding and transporting fatty acids and endogenous hormones, albumin is the ideal carrier for peptide drugs). Due to the large size of the albumin-peptide complex, the diffusion rate in the tissue and on the capillary wall is usually reduced after injection [2].
In addition, the lipidation modification leads to an increase in hydrophobicity of the peptide drug molecules, which enhances the self-association between the molecules, which decreases the absorption rate in the subcutaneous tissue and leads to a slower diffusion rate through the tissue and capillary wall[3] . Proteins with a molecular weight greater than 16 kDa can also be absorbed via the lymphatic route[4]. Lipidated peptides entering the systemic circulation enhance the pharmacokinetic profile of peptide drugs due to reversible, high affinity binding to albumin, reducing renal clearance of large size peptides and decreasing the rate of distribution to extravascular compartments[5] .
The long alkyl chain form of mono fatty acids has a long hydrocarbon chain and a terminal carboxylic acid group, and its affinity for albumin binding increases with the length of the alkyl chain within a certain range [6]. Later, fatty diacid types were introduced to provide a stronger affinity for albumin. This may result in longer half-lives due to the introduction of additional carboxylic acid groups [7]. Albumin affinity is closely related to the length of the fatty mono- or diacid. 1,18-Octadecanedioic acid (C18 diacid) and 1,20-eicosanedioic acid (C20 diacid) are the fatty acids with the highest affinity for albumin tested to date ( Figure 1) [8].
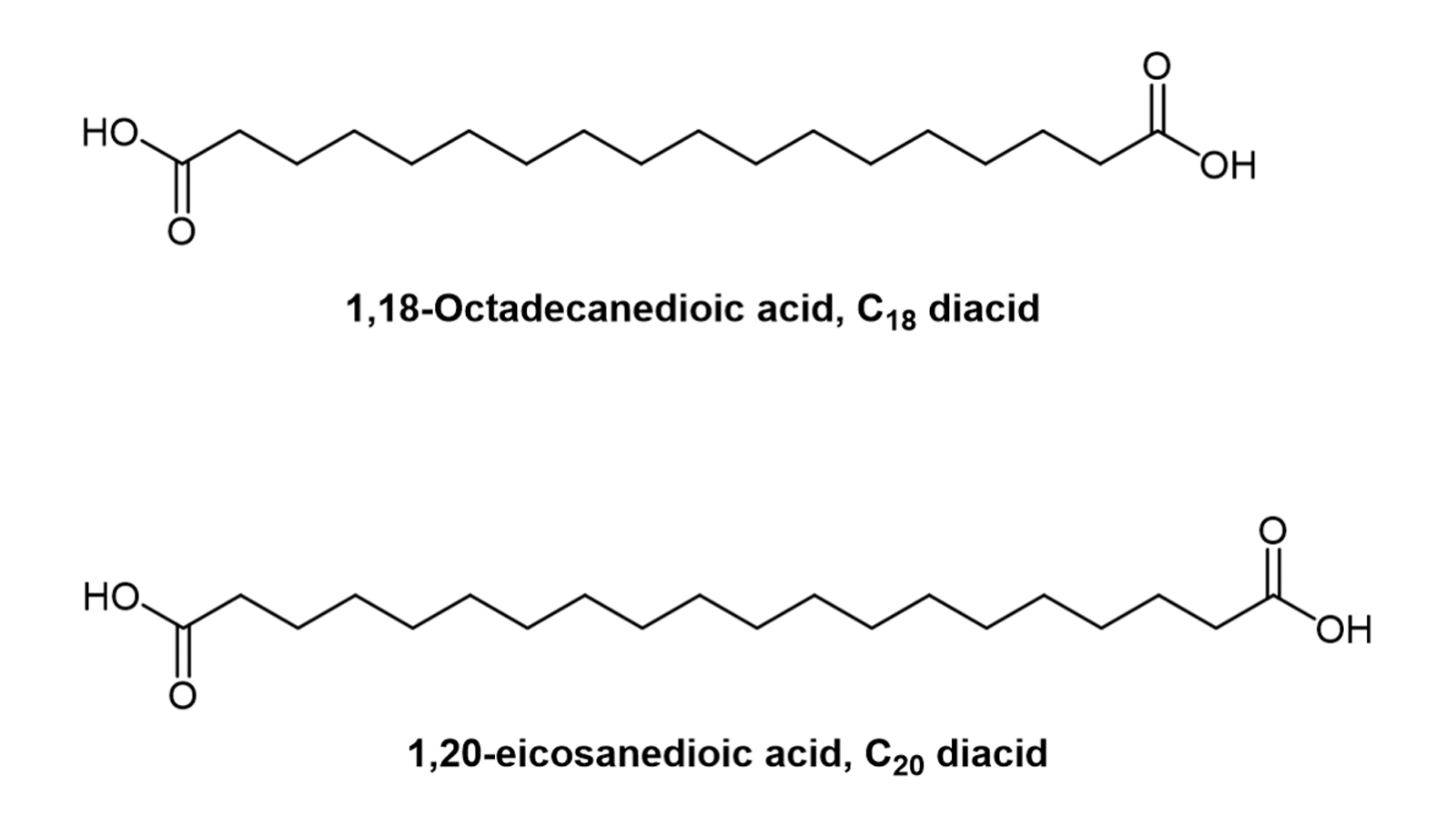
Figure 1. Chemical structures of C18 and C20 diacids
The hydrophobicity of fatty monoacids is higher than that of fatty diacids, which can affect the solubility, receptor pharmacology and biophysical properties of the derived molecule. Derivatization of fatty monoacids will allow the derived protein to bind to the cell membrane and promote endocytosis [9]. In contrast, fatty diacids have uncondensed carboxyl groups that increase solubility, and fatty diacid-derived peptides are less likely to bind to cell membranes and endocytose [10]. Therefore, in vivo efficacy is often better maintained with fatty diacids because the target peptide or protein is not lost or internalised due to hydrophobic interactions with the cell surface. The type of fatty acid attached may affect receptor specificity. The in vitro and in vivo efficacy of a peptide or protein may be affected to varying degrees depending on whether it is a fatty mono acid or a fatty diacid that is attached.
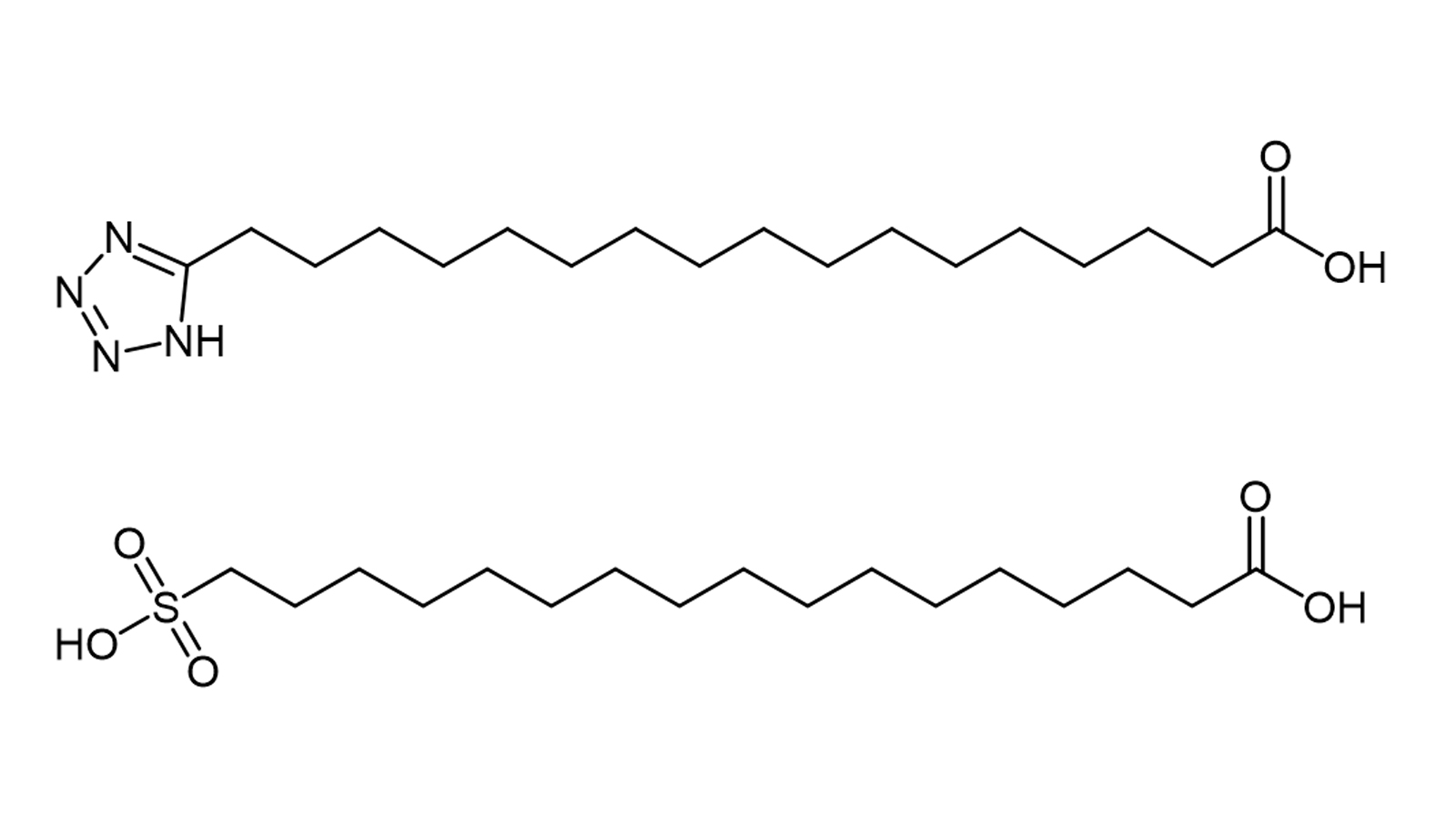
Figure 2. Chemical structures of tetrazole and sulfonic acid analogues of C18 diacids
The most important effect of the distal free carboxyl group in adipic acid is that it confers a higher albumin binding affinity and therefore a longer half-life. In addition it has been reported that adipic acid analogues with a distal sulfonyl or tetrazolium group (both electron isostere of carboxylic acids) (Figure 2), bind with high affinity to albumin and prolong the action curve of the peptide or protein [11]. For some peptides, however, these electron equivalents do not appear to add any major advantage over the carboxyl type diacids. It seems that the underlying causes affecting albumin binding need to be further investigated.
The position of the fatty acid in the protein or peptide backbone is important for optimal potency and half-life. This is especially true when the fatty acid is directly modified to the main chain (e.g. by acylating the amino group of the lysine side chain). However, when a spacer structure such as glutamate is inserted between the fatty acid and the peptide backbone, the impact of the peptide backbone on binding to albumin is likely to be less. In one study, the X-ray structure in a fatty acid-derived hGH (human growth hormone) complex with human albumin was found to show two binding sites, neither involving the peptide backbone [12].
In addition to fatty acid type and modification sites, the junction and arm linker structures (arm linker) connecting the lipid to the peptide have a significant impact on the pharmacokinetic profile of the derived peptide, the details of which are not detailed in this paper for reasons of space.
The most well-known lipidated peptides are semaglutide, liraglutide and tirzepatide.
.png)
Figure 3. Structural formula of semaglutide
Semaglutide (Figure 3) is a GLP-1 analogue, a GLP-1 receptor agonist peptide targeting type 2 diabetes and obesity. The lysine at position 26 of semaglutide is modified by stearic acid diacid (C18 diacid), which increases its binding to blood albumin, thereby prolonging its presence in the circulation. Between the stearic acid diacid and lysine, there is also a spacer consisting of glutamic acid and 2-(2-(2-aminoethoxy)ethoxy)acetic acid dimer. semaglutide has a half-life in the blood of approximately 7 days (165-184 hours) [13]. It can be administered by weekly subcutaneous injection (Ozempic®) or orally once daily (Rybelsus®).
.png)
Figure 4. Structural formula of liraglutide (Liraglutide)
Liraglutide (Victoza®, Saxenda®) is a GLP-1 analogue used for the treatment of type 2 diabetes, obesity and the prevention of cardiovascular complications associated with diabetes. The Lys26 side chain of liraglutide is coupled to a C-16 fatty acid (also known as palmitic acid or soft fatty acid), linked by a glutamate spacer (Figure 4). This palmitic acid modification allows liraglutide to bind reversibly to albumin in subcutaneous tissue and blood and to be released slowly over time. Compared to GLP-1, binding to albumin results in slower degradation and reduced elimination of liraglutide from the renal circulation.
.png)
Figure 5. Structural formula of tirzepatide
Tirzepatide (Mounjaro®), Eli Lilly's type 2 diabetes peptide, is a GLP-1/GIP receptor co-agonist administered by subcutaneous injection once weekly. Telbopeptide is an analogue of human GIP (glucose-dependent insulinotropic peptide) with a C20 fatty acid diacid fraction attached to optimise the uptake and metabolism of the compound [14]. The fatty diacid fraction (eicosanoic acid) is attached to the side chain of the lysine residue via glutamic acid and two (2-(2-aminoethoxy)ethoxy)acetic acid units (Figure 4). This lipidation modification produces a longer half-life and extends the time between doses, with a half-life of approximately 5 days for telopeptide [15].
The choice of pharmacokinetic prolongation technique for a peptide or protein drug depends on the intended route of administration and the associated route of elimination. The lipidation technique may be useful in many cases because of its good chemical profile relative to polyethylene glycol derivatization. In addition, the technique has the potential to influence the rate of absorption following subcutaneous administration and the rate of elimination by the various routes of clearance.
Degludec (insulin degludec) is an analogue derived with C16 fatty diacid, which forms a dimerised hexamer (di-hexamer) in the formulation buffer. Once injected into the subcutaneous tissue, the di-hexamer forms a poly-hexamer.
Liraglutide and semaglutide are glucagon-like peptide 1 (GLP-1) analogues. The lysine side chain of liraglutide is coupled to a C16 aliphatic monoacid (palmitic acid); the lysine side chain of semaglutide is modified by a C18 aliphatic diacid. Liraglutide forms a heptamer in the formulation buffer and once injected, the liraglutide monomer slowly dissociates from the heptamer and binds to albumin. Some of the dissociated liraglutide monomers, may be anchored in the cell membrane via monoacid side chains.
For both deglutethimide and liraglutide, the rate-limiting step in the distribution from subcutaneous tissue through the blood into the tissue is the release of the monomer, resulting in flip-flop kinetics. Facilitated by adiponectin, semaglutide binds strongly to albumin and protects it from protease degradation. In the bloodstream, the albumin-shaded stigmaglutide forms a reservoir from which it is slowly released. Non-albumin-binding molecules can be eliminated by non-receptor-mediated pathways, or they can bind to target receptors to trigger their biological effects and then be eliminated. Peptide-albumin complexes can also bind to target receptors and trigger biological effects if spatial parameters allow.
The peptide-albumin complex can also bind to the neonatal Fc receptor (FcRn) via the albumin fraction and in this way enable recycling and reuse, thus further extending the cycle time of the derived molecule.
Lipid derivatization is a clinically proven chemical modification technique that can be used to optimize the pharmacological properties of peptide and protein drugs. The technique provides a way to extend the inherently short half-life of natural molecules to allow for daily, weekly or even longer dosing intervals.
In addition, fatty acid derivatisation can be used to facilitate the oral delivery of biological drugs and potentially alter their distribution in tissues. Peptide lipidation is safe and effective, it can also be optimised by various medicinal chemistry methods and allows for cost-effective mass production. In conclusion, lipidation derivatization has been successfully applied in pharmaceutical engineering and is likely to play an increasingly important role in advancing peptide drug development.
Please contact us to remove any infringement.